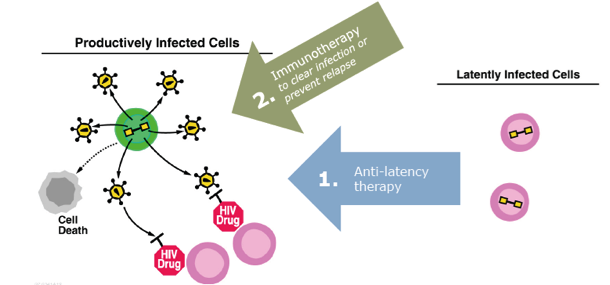
Stepwise Approach: Reverse latency and then clear the virus
Curing HIV infection has been a difficult challenge primarily due to the persistence of viral reservoirs that remain in the body even during uninterrupted, long-term antiretroviral therapy. These reservoirs exist as HIV genomes (proviruses) that integrate into the cellular DNA of extremely long-lived immune cells known as T cells. Within these cells, the provirus can remain silent and undetectable for decades in a state known as viral latency. During this time, the provirus enjoys sanctuary from drug therapy and from the host immune system. If therapy is interrupted at any point, the provirus can reactivate, resulting in viral rebound within the body. Without intervention, increased viral loads can lead to depletion of host T cells, impairment of the immune system, progression to AIDS, and ultimately death. Targeted approaches are being explored to reverse latency, induce viral antigen expression within formerly latently infected cells, and to pair the latency reversing agents with immune clearance mechanisms to eradicate persistent infection. One of our research goals is to understand how to reverse latency so that the virus can be cleared from the body.
UNC HIV Cure Center has a rich and diverse latency reversal research portfolio with two major themes. The first is the discovery, development and testing of latency reversing agents. Libraries of compounds are being screened to assess their ability as single agents or in combination to reverse latency. Hits from these screens are moved forward into in vitro cell line and primary cell models and if warranted, in vivo models The second is the identification of new targets that can be exploited to selectively re-activate HIV. Cellular pathways that enforce HIV latency are good targets for agents that reverse latency and we are exploring these pathways to identify druggable targets.
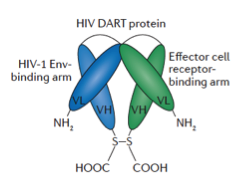
Immune Clearance of HIV Using a Dart Molecule
Ferrari, Nature Reviews Drug Discovery, 2016
Targeted approaches to reverse latency are being explored so that viral antigen is expressed by a formerly latently infected cell and becomes vulnerable to immune clearance mechanisms. We are exploring antibodies, engineered antibody-like Dual Affinity Re-Targeting (DARTs) molecules, vaccines, and immune checkpoint modulation strategies to clear the HIV virus from the body and the UNC HIV Cure Center has a rich and diverse clearance research portfolio centered around these major approaches.
Therapeutic antibodies that can specifically direct immune clearance of HIV-infected cells are a relatively new area of HIV cure research and may be useful and important tools for clearance. DARTs are designed to redirect the activity of T-cells to target the cells infected with HIV. Novel immune-augmenting strategies, such as adjunctive treatment with immune checkpoint inhibitors, might reawaken an extant but exhausted or diminished immune response and facilitate clearance of viral reservoirs. T-cell vaccines are also being tested for their ability to redirect the immune response to enhance clearance. These clearance strategies are being tested alone and in combination with latency reversing agents in in vitro and in vivo models.
-
The replication-competent HIV-1 latent reservoir is
primarily established near the time of therapy initiation.
Sci Transl Med 2019 Nov; 11(513)
-
Precision mouse models with expanded tropism for
human
pathogens.
Nat. Biotechnol. 2019 Nov; 37(10)
-
Publisher Correction: The immune cell landscape in
kidneys
of patients with lupus nephritis.
Nat. Immunol. 2019 Sep; 20(10)
-
Dual effects of the novel ingenol derivatives on the
acute
and latent HIV-1 infections.
Antiviral Res. 2019 Aug; 169
-
Deep latency: A new insight into a functional HIV
cure.
EBioMedicine 2019 Jul; 45
-
The immune cell landscape in kidneys of patients
with lupus
nephritis.
Nat. Immunol. 2019 Jul; 20(7)
-
Gut Microbiome Alterations During HIV/SIV Infection:
Implications for HIV Cure.
Front Microbiol 2019 Jun; 10
-
A Reproducible, Objective Method Using MitoTracker®
Fluorescent Dyes to Assess Mitochondrial Mass in
T Cells by Flow Cytometry.
Cytometry A 2019 Nov; 95(4)
-
In-vivo administration of histone deacetylase
inhibitors
does not impair natural killer cell function in HIV+
individuals.
AIDS 2019 Nov; 33(4)
-
Human cytomegalovirus-vectored vaccines against
HIV.
Curr Opin HIV AIDS 2019 Nov; 14(2)
-
Harnessing CD8
Front Immunol 2019 Nov; 10
-
HIV-Specific, Ex Vivo Expanded T Cell Therapy:
Feasibility,
Safety, and Efficacy in ART-Suppressed HIV-Infected Individuals.
Mol. Ther. 2019 Aug; 26(10)
-
Single-Cell Analysis of Quiescent HIV Infection
Reveals Host
Transcriptional Profiles that Regulate Proviral Latency.
Cell Rep 2019 Nov; 25(1)
-
γδ T cells: an immunotherapeutic approach for HIV
cure
strategies.
JCI Insight 2019 Nov; 3(12)
-
Interleukin-15-Stimulated Natural Killer Cells Clear
HIV-1-Infected Cells following Latency Reversal
J. Virol. 2018 Jun; 92(12)
-
Correction to: HIV Persistence on Antiretroviral
Therapy and
Barriers to a Cure.
Adv. Exp. Med. Biol. 2019 Nov; 1075
-
HIV Persistence on Antiretroviral Therapy and
Barriers to a
Cure.
Adv. Exp. Med. Biol. 2019 Feb; 1075
-
Vorinostat Renders the Replication-Competent Latent
Reservoir of Human Immunodeficiency Virus (HIV) Vulnerable to
Clearance by
CD8 T Cells.
EBioMedicine 2018 May; 23
-
Chromatin Regulation and the Histone Code in HIV
Latency
.
Yale J Biol Med 2017 Oct; 90(2)
-
Interrupting antiretroviral treatment in HIV cure
research:
scientific and ethical considerations.
J Virus Erad 2019 Nov; 3(2)
-
Towards an HIV Cure: a View of a Developing
Field.
J. Infect. Dis. 2019 Feb; 215(suppl_3)
-
Proviral Latency, Persistent Human Immunodeficiency
Virus
Infection, and the Development of Latency Reversing Agents.
J. Infect. Dis. 2017 Jul; 215(suppl_3)
-
Quantification of the Latent HIV-1 Reservoir Using
Ultra
Deep Sequencing and Primer ID in a Viral Outgrowth Assay.
J. Acquir. Immune Defic. Syndr. 2017 Jun; 74(2)
-
Benzotriazoles Reactivate Latent HIV-1 through
Inactivation
of STAT5 SUMOylation.
Cell Rep 2017 Nov; 18(5)
-
Detection of human immunodeficiency virus RNAs in
living
cells using Spinach RNA aptamers.
Virus Res. 2017 Dec; 228
-
HIV antibodies for treatment of HIV infection.
Immunol. Rev. 2017 Aug; 275(1)
-
Transcriptomic Analysis Implicates the p53 Signaling
Pathway
in the Establishment of HIV-1 Latency in Central Memory CD4 T Cells
in an In
Vitro Model.
PLoS Pathog. 2017 May; 12(11)
-
HIV Latency-Reversing Agents Have Diverse Effects on
Natural
Killer Cell Function.
Front Immunol 2019 Nov; 7